随着科技的进步和医疗技术的不断创新,无创血糖仪正逐步迈向更精准、更智能、更易于操作的新阶段。它不仅仅是一个测量工具,更是连接患者、家属与医疗专业人员的桥梁,通过远程监控、数据分析等功能,构建起全方位、个性化的糖尿病管理体系。未来,无创血糖仪的应用将进一步拓宽,有望成为家庭健康监测、临床决策支持及公共卫生管理的重要工具,为全球数以亿计的糖尿病患者带来福音,开启糖尿病管理的新篇章。
With advancements in technology and continuous innovation in medical techniques, non-invasive blood glucose meters are gradually evolving towards a new stage of higher accuracy, intelligence, and ease of use. They are not just measurement tools but also bridges connecting patients, families, and medical professionals, establishing a comprehensive and personalized diabetes management system through remote monitoring and data analysis. In the future, the application of non-invasive blood glucose meters will further expand, potentially becoming important tools for home health monitoring, clinical decision-making support, and public health management, bringing blessings to hundreds of millions of diabetes patients worldwide and ushering in a new chapter in diabetes management.

1、分类编码(The classification code.):
2、预期用途(Intended Use) :
3、产品原理(Product Principle):
无创血糖仪的产品原理主要基于以下几种技术,通过非侵入性的方式测量血糖水平,旨在提供便捷、舒适的血糖监测体验:
The product principle of non-invasive blood glucose meters is mainly based on the following technologies, which measure blood glucose levels in a non-invasive manner, aiming to provide a convenient and comfortable blood glucose monitoring experience:
1)光谱测量技术(Spectral measurement technology)
2)生物电极技术(Bio-electrode technology)
3)其他间接测量技术(Other indirect measurement technologies)
4)光声成像技术+AI智能技术(Photoacoustic imaging technology + AI intelligent technology)
4、产品优势(Product Advantages):
1)便捷舒适(Convenient and comfortable)
2)快速准确(Fast and accurate)
3)智能互联(Intelligent connectivity)
4)节省成本(Cost-saving)
5)适用广泛(Widely applicable)
5、无创血糖监测产品宜参考的现行有效标准(The currently effective standards that non-invasive blood glucose monitoring products should refer to.)
标准编号 (Standard Numbers) | 标准名称 (Standard Names) |
GB 9706.1 | 医用电气设备 第1部分:基本安全和基本性能的通用要求 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance |
GB 9706.15-2008 | 医用电气设备 第1-1部分:安全通用要求并列标准:医用电气系统安全要求(如适用) Medical electrical equipment - Part 1-1: General requirements for safety - Collateral standard: Safety requirements for medical electrical systems (if applicable) |
GB/T 14710 | 医用电器环境要求及试验方法 Environmental requirements and test methods for medical electrical equipment. |
GB/T 16886.1 | 医疗器械生物学评价 第1部分:风险管理过程中的评价与试验 Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process. |
GB/T 16886.5 | 医疗器械生物学评价 第5部分:体外细胞毒性试验 Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. |
GB/T 16886.10 | 医疗器械生物学评价 第10部分:刺激与迟发型超敏反应试验 Biological evaluation of medical devices - Part 10: Tests for irritation and delayed-type hypersensitivity. |
GB/T 16886.12 | 医疗器械生物学评价 第12部分:样品制备与参照样品 Biological evaluation of medical devices - Part 12: Sample preparation and reference samples. |
GB/T 16886.18 | 医疗器械生物学评价 第18部分:材料化学表征 Biological evaluation of medical devices - Part 18: Chemical characterization of materials. |
GB/T 14233.2 | 医用输液、输血、注射器具检验方法 第2部分:生物学试验方法 Test methods for medical infusion, transfusion, and injection equipment - Part 2: Biological test methods. |
GB/T 42062 | 医疗器械风险管理对医疗器械的应用 Application of risk management to medical devices. |
GB/T 21416 | 医用体温计(如适用) Medical thermometer (if applicable). |
YY 0785 | 临床体温计连续测量的电子体温计性能要求(如适用) Performance requirements for electronic clinical thermometers for continuous measurement (if applicable). |
YY 9706.102 | 医用电气设备 第1-2部分:基本安全和基本性能的通用要求并列标准:电磁兼容要求和试验 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility requirements and tests. |
或or | 或or |
YY 0505 | 医用电气设备 第1-2部分:安全通用要求并列标准:电磁兼容要求和试验 Medical electrical equipment - Part 1-2: General requirements for safety - Collateral standard: Electromagnetic compatibility requirements and tests. |
YY 9706.108 | 医用电气设备 第1-8部分:基本安全和基本性能的通用要求并列标准:通用要求,医用电气设备和医用电气系统中报警系统的测试和指南(如适用) Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral standard: Tests and guidance for alarm systems in medical electrical equipment and medical electrical systems (if applicable). |
或or | 或or |
YY 0709 | 医用电气设备 第1-8部分:安全通用要求并列标准 医用电气设备和医用电气系统中报警系统的测试和指南(如适用) Medical electrical equipment - Part 1-8: General requirements for safety - Collateral standard: Tests and guidance for alarm systems in medical electrical equipment and medical electrical systems (if applicable). |
YY 9706.111 | 医用电气设备 第1-11部分:基本安全和基本性能通用要求-并列标准:在家庭护理环境中使用的医用电气设备和医用电气系统的要求 Medical electrical equipment - Part 1-11: General requirements for basic safety and essential performance - Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment. |
YY 9706.256 | 医用电气设备 第2-56部分:用于体温测量的临床体温计的基本安全和基本性能专用要求 Medical electrical equipment - Part 2-56: Particular requirements for the basic safety and essential performance of clinical thermometers for body temperature measurement. |
YY 9706.261 | 医用电气设备 第2-61部分:脉搏血氧设备的基本安全和基本性能专用要求(如适用) Medical electrical equipment - Part 2-61: Particular requirements for the basic safety and essential performance of pulse oximetry equipment (if applicable). |
或or | 或or |
YY 0784 | 医用电气设备 医用脉搏血氧仪设备基本安全和主要性能专用要求(如适用) Particular requirements for the basic safety and essential performance of medical electrical equipment - Medical pulse oximeters (if applicable). |
6、临床试验要求(Clinical trial requirements.):
7、注册路径(Registration pathway.):
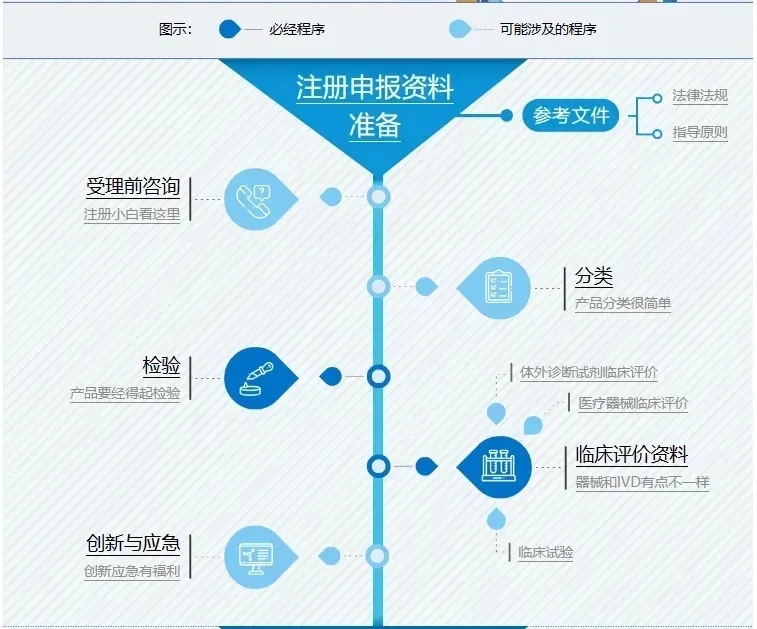
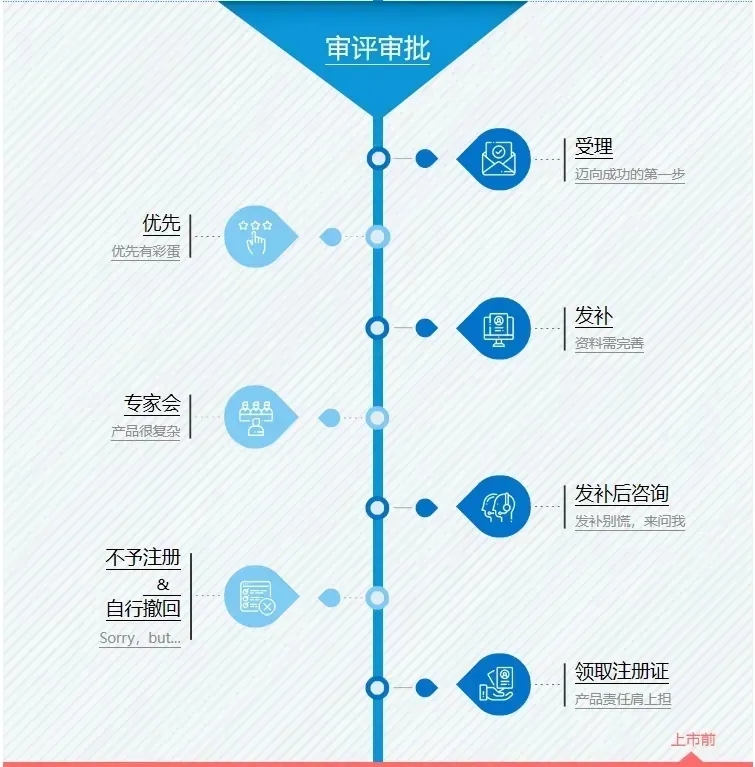
Hom Medicine excels in the field of medical device clinical trials, not only possessing profound professional knowledge but also having a mature and experienced team, as well as extensive clinical experience with non-invasive blood glucose meters. We specialize in providing enterprises with professional testing, registration, and clinical trial services, aiming to support their development and offer comprehensive technical assistance.
鸿盟医学
医疗器械全球注册咨询、医疗器械临床试验、医疗器械质量管理体系(ISO13485/GMP/QSR)、医疗器械可用性研究、
医疗器械法规培训众多的专业技术服务咨询机构
微信号:hom_medical、18018710006 联系电话:18018716006
 鸿盟标准技术(深圳有限公司)
鸿盟标准技术(深圳有限公司)